Abstract
Introduction- Next generation flow (NGF) is one of the approaches for testing multiple myeloma (MM) minimal residual disease (MRD) over conventional response assessments. Actually, bone marrow (BM) is the preference site of evaluation because of its sensitivity. Because of its invasively technic, other possible sites for MRD evaluation outside the BM have been studied. In the present study we analyzed the MRD between the BM and the hematopoietic stem cell collected product (HSC product), once the concentration of plasma cell in the HSC product could be higher than peripheric blood sample.
Aims- To compare MRD quantification of plasma cell between BM and HSC product after induction from Newly Diagnosed MM(NDMM) Transplant Eligible (TE) patients (pts) exposed to daratumumab, cyclophosphamide, thalidomide and dexamethasone (Dara-CTD) protocol.
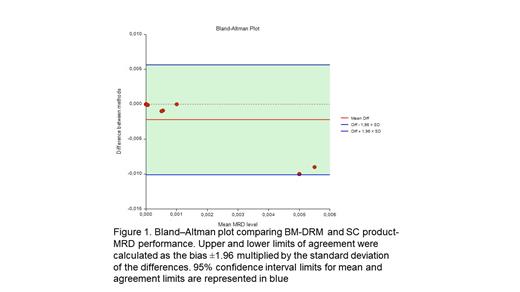
Methods- The SC product and BM samples were collected after four 28 days cycles of induction therapy from pts treated with Dara-CTd protocol described before by (Crusoe E. et al. Blood 2020; 136 (supplement 1): 17-18). MRD was evaluated by next-generation flow (NGF) based in the EuroFlow® protocol. EuroFlow standards was used to identify clonality and aberrant PC immune phenotype, consisting by EuroFlow 8-color 2-tube method (MM MRD kit, Cytognos, Salamanca), with the acquisition of 5 million events each tube and then merged into a single analysis tube on approximately 10 million events. Plasma cells were identified by CD38 multiepitope and CD138. Other markers were used to detect abnormal phenotypes. For comparison of MRD results, Bland-Altman plot comparing BM-MRD and HSC product-MRD was performed.
Results- The first pts was enrolled in November 2018. A total of 24 pts were included, the median age was 60 (range 37- 67 years), 23 (92%) were non-white, 5 (21%) had an R-ISS = 1, 12 (54%) had an R-ISS = 2 and 4 (16%), an R-ISS = 3. Six (25%) pts had high-risk chromosomal abnormalities [del17p, t(4;14) or t(14;16)]. To date, all pts have completed induction and 20 have received transplant. Regarding response rates, after the end of induction (cycle 4), 19 (90%) of the pts obtained > PR and 8 (38%) obtained >VGPR, including three MRD negativity by NGF. 19 pts were analyzed for MRD. Negative MRD in sensitivity <10 -5, >=10 -5 and <10 -4, >=10 -4 evaluated in bone marrow was 4/19(21%), 4/19(21%), 11/19(58%) respectively. Negative MRD in sensitivity <10 -5, >=10 -5 and <10 -4, >=10 -4 evaluated in the HSC product was 13/19(68%), 3/19(16%), 3/19(16%) respectively. Median bone marrow sensitivity 10 -4 lower quartile 10 -5 upper quartile 10 -3. Normal distribution of the differences between BM and SC product MRD was first assessed (Kolmogorov-Smirnov's p < 0.001, n = 19).
Discussion-Conclusions- The use of HSC product could enhance the plasma cell concentration and may be an alternative and attractive method for MRD detection that diminished the invasiveness of repetitive bone marrow aspirations and tackling the heterogeneity distribution of MM cells. In this preliminary data the sample size did not allow to show a direct correlation between BM and HCS product. A larger sample would be needed to confirm the hypothesis.
Hungria: Amgen, BMS, Celgene, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings/travel ; Abbvie: Honoraria; Sanofi: Honoraria, Other: Support for attending meetings/travel ; Takeda: Honoraria. De Queiroz Crusoe: Janssen: Research Funding.